GSK Gets FDA Nod for Measles, Mumps & Rubella Vaccine, Priorix
GSK plc GSK announced that the FDA has approved Priorix (Measles, Mumps and Rubella Vaccine, Live) for active immunization for the prevention of measles, mumps and rubella (“MMR”) in individuals 12 months of age and older.
Per the Centers for Disease Control and Prevention (“CDC”), people should get an MMR vaccine as measles, mumps and rubella are acute and highly-contagious viral diseases
Per the press release, Priorix is scheduled to be on the agenda for the June CDC Advisory Committee on Immunization Practices meeting.
The safety of Priorix was investigated across six studies in 12,151 participants who received at least one dose of the vaccine.
Priorix can be administered as a first dose followed by a second one. The vaccine can also be administered as a second dose for individuals who have previously received the first dose of another MMR-containing vaccine.
Following the latest FDA nod, Priorix is now likely to be available in the United States for the first time, giving healthcare professionals another MMR vaccine choice in the country.
Till date, more than 800 million doses of Priorix have been distributed in more than 100 countries worldwide, including all European countries, Canada, Australia and New Zealand.
Shares of GSK have lost 3.1% so far this year against the industry’s increase of 4.1%.
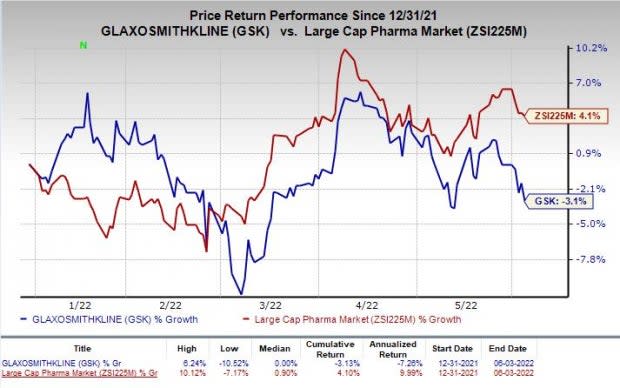
Image Source: Zacks Investment Research
We note that the approval of Priorix will boost GSK’s vaccine portfolio. The company already boasts of a strong portfolio of vaccines approved for various indications.
In the first quarter of 2022, vaccine sales gained 36% at constant exchange rate, mainly driven by strong demand recovery in Shingrix (shingles vaccines) sales.
In 2022, GSK expects vaccine sales to grow at low-teens percentage points, excluding pandemic solutions.
Last week, GSK entered into a definitive agreement to acquire privately held company, Affinivax, Inc., for an upfront payment of $2.1 billion. GSK will also pay up to $1.2 billion in potential development milestones. The transaction is expected to close in the third quarter of 2022.
With the acquisition, GSK is looking to boost its vaccines pipeline and get access to a new, potentially disruptive technology to build a strong portfolio of innovative vaccines and specialty medicines.
Zacks Rank & Stocks to Consider
GSK currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector are Leap Therapeutics, Inc. LPTX, Anavex Life Sciences Corp. AVXL and Precision BioSciences, Inc. DTIL, all carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Zacks Consensus Estimate for Leap Therapeutics’ loss per share has narrowed 11.1% for 2022 and 5.9% for 2023 in the past 60 days.
Earnings of Leap Therapeutics have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion. LPTX delivered an earnings surprise of 1.92%, on average.
Anavex Life Sciences’ loss per share estimates narrowed 6.6% for 2022 and 4.3% for 2023 in the past 60 days.
Earnings of Anavex Life Sciences have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions. AVXL delivered an earnings surprise of 0.48%, on average.
Precision BioSciences’ loss per share estimates narrowed 21.7% for 2022 and 31.4% for 2023 in the past 60 days.
Earnings of Precision BioSciences have surpassed estimates in each of the trailing four quarters. DTIL delivered an earnings surprise of 76.15%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Anavex Life Sciences Corp. (AVXL) : Free Stock Analysis Report
Leap Therapeutics, Inc. (LPTX) : Free Stock Analysis Report
Precision BioSciences, Inc. (DTIL) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research


