Coronavirus update: CDC eyes small groups as US cases spike; Sanofi announces pre-clinical vaccine data
The U.S. is in the throes of a wave of new COVID-19 diagnoses across the country, which is expected to worsen as flu season sets in.
Seven-day rolling averages have increased in 44 out of 50 states, stirring the nation’s top health official to increase warnings about social distancing measures as fatigue sets in among the population. The current rise in cases, and efforts to contain them, mark a critical window that is closing as winter arrives, and ahead of when a vaccine breakthrough is expected.
In a recent interview, Dr. Anthony Fauci, the nation’s top infectious disease expert, told CBS that people “should be very careful and prudent about social gatherings,” adding he planned to have a virtual Thanksgiving with his children this year.
On a recent governors call, U.S. Center for Disease Control and Prevention (CDC) Director Dr. Robert Redfield said that family gatherings are the reason for continued spread. As Thanksgiving approaches, the official noted that “what we're seeing as the increasing threat right now is actually acquisition of infection through small household gatherings.”
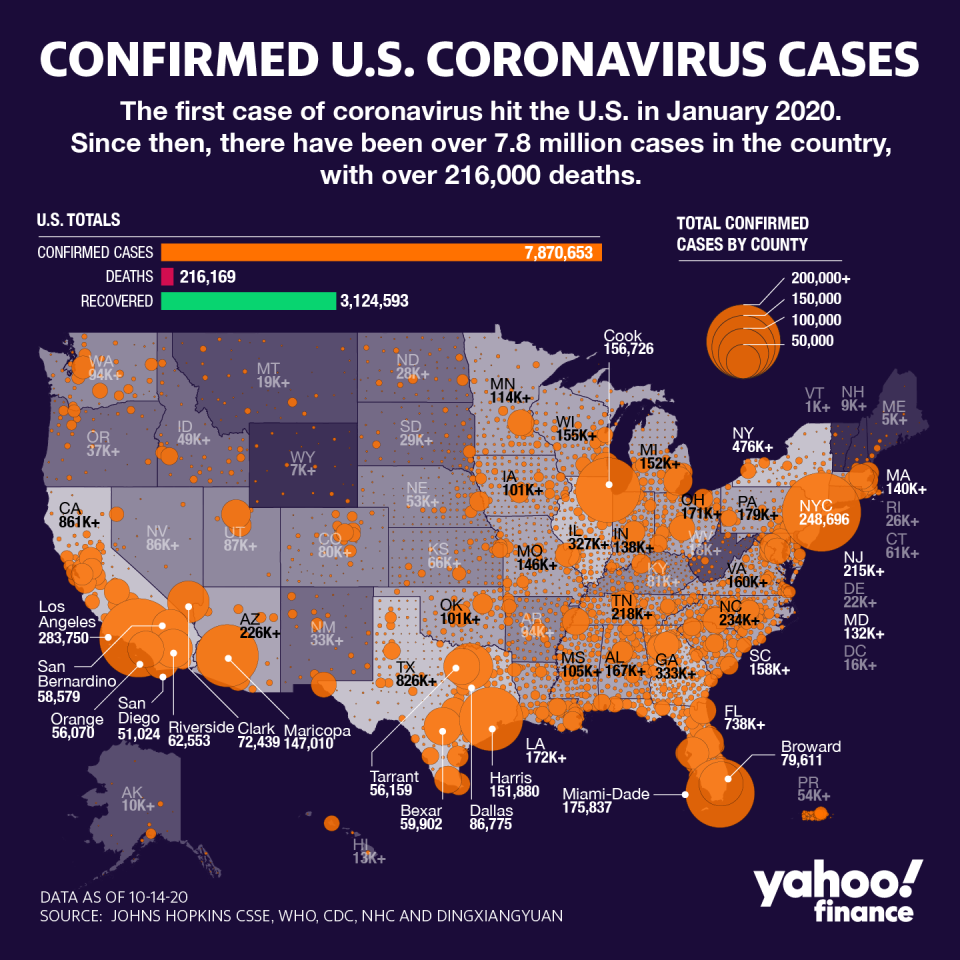
The global toll has now surpassed 38.5 million, with nearly 1.1 million deaths. In the U.S., nearly 8 million have tested positive and the death toll is approaching 217,000. Yet while positive diagnoses are on the rise domestically, hospitals in previously hard-hit epicenters remain well below crisis levels seen during the spring, and fatality rates have also been tempered.
However, Europe has overtaken the U.S. in total new cases, and has reverted to stricter lockdowns across the continent — with bars and restaurants closing back up.
While it is unclear what level of restrictions will be re-imposed in the U.S., schools have already shifted to remote in some areas — like New York, which initially sought to fully re-open schools. All while the country is in the final weeks of the election cycle for the 2020 presidential election, and a stimulus package remains stuck in Congress — leading to selling on Wall Street.
The World Health Organization has repeatedly warned against long-term lockdowns, noting its negative impact on society and the economy, yet comments from a recent WHO advisor on ending restrictions sparked controversy.
David Nabarro, an advisor to the WHO director, said the virus is only beginning its relationship with the human race, and has only affected 10% of the human population.
“We in the World Health Organization do not advocate lockdowns as a primary means of control for this virus,” he said, adding that it was only supposed to be used to “buy time” to bolster the health care system.
His words reflect those of current director general Tedros Adhanom Ghebreyesus, who has repeatedly cited the need to limit lockdowns as a last resort to help curb the spread with the goal to lean more heavily on testing and tracing as well as social distancing measures.
In March, he said that asking people to stay at home is, “buying time,” but it wasn’t enough. Ghebreyesus previously embraced the idea of “a slow, steady lifting of lockdowns” to stimulate economies, while warning against “lurching from lockdown to lockdown.”
Meanwhile, hopes for a vaccine by election day have been all but extinguished, while some trials have hit snags. Still, the CDC anticipates a vaccine by the end of the year.
Currently, the earliest possible results of a vaccine trial will be in November. An advisory council is set to meet on Oct. 22 to set the final parameters for safety and efficacy guidelines for the U.S. Food and Drug Administration (FDA) to use in order to authorize any vaccine.
Sanofi (SNY) is the latest to produce data Thursday, highlighting a strong response from its mRNA vaccine candidate in a pre-print report.
The technology being used by the company is similar to frontrunners Moderna (MRNA) and Pfizer (PFE) with BioNTech (BNTX). Meanwhile AstraZeneca (AZN) and Johnson & Johnson (JNJ), both of which are using a version of adenovirus technology, have had to halt their trials in the U.S.
More from Anjalee:
Redfield: CDC 'preparing earnestly' for vaccine in November, December
India's tech giants navigate 'literally spiking' WFH demand, H1-B fears in coronavirus era
How protests spurred Corporate America into action on race, inequality
Read the latest financial and business news from Yahoo Finance
Follow Yahoo Finance on Twitter, Facebook, Instagram, Flipboard, SmartNews, LinkedIn, YouTube.



