China's Sinovac coronavirus vaccine trial suspended in Brazil after participant dies
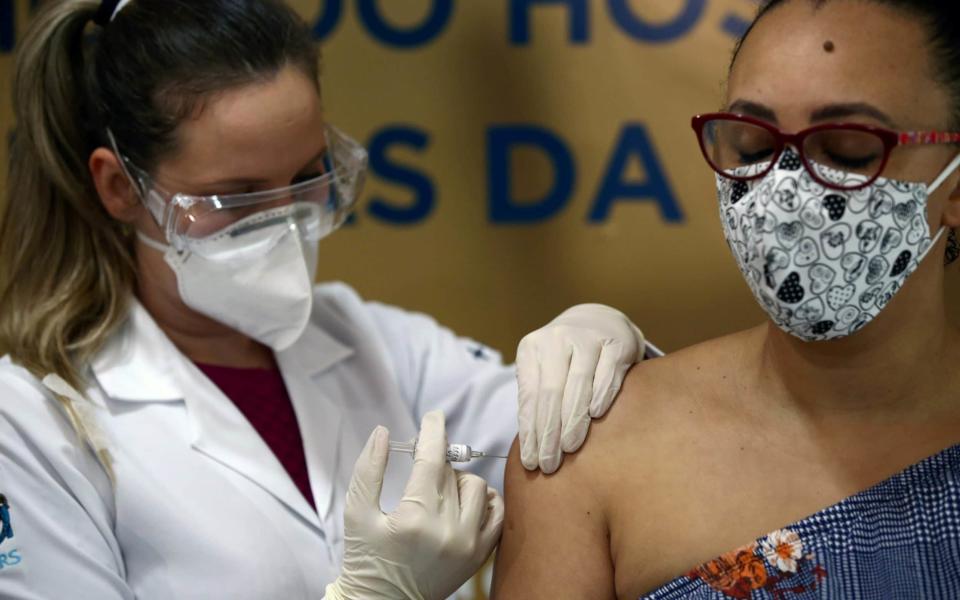

Brazil has suspended clinical trials for China's coronavirus vaccine after a participant died.
Instituto Butantan, the research centre in Sao Paulo developing the vaccine in partnership with Sinovac, a private Chinese firm, said it was surprised by the decision.
Dimas Covas, director of the institute, told Brazilian media that a study volunteer had died, though the death was not linked to ongoing trials.
“As there are more than 10,000 volunteers at this moment, deaths can occur,” said Mr Covas. “It’s a death that has no relation with the vaccine and as such it is not the moment to interrupt the trials.”
The state government of Sao Paulo, where the trial is being run, said the death of a trial volunteer had been registered as a suicide and was being investigated.
Brazil’s health regulator Anvisa said in a statement late on Monday that an "adverse, serious event" had occurred on Oct 29, but it didn’t say there had been a death. Nor did it elaborate on what happened or why it was revealing this more than a week later.
Anvisa later said the initial information it received from Butantan had not specified that the death was a suicide.
"We had no choice but to suspend the trials given the event," the head of the agency Antônio Barra Torres said.
Sinovac said it had been in touch with Butantan, and learned the director, Mr Covas, "believed that this serious adverse event is not related to the vaccine."
The Chinese company remains in contact with Brazil and reiterated its confidence in the safety of its vaccine.
Vaccine studies have been suspended in recent months after serious adverse events to allow experts to investigate. Suspensions have impacted vaccines jointly developed by AstraZeneca and the University of Oxford, and another by American firm Johnson & Johnson, though trials later resumed after scientists deemed it safe to continue.
In Brazil, the second hardest-hit by the pandemic with more than 160,000 deaths, access to the coronavirus vaccine has been politicised by a rivalry between president Jair Bolsonaro and Sao Paulo state governor Joao Doria.
Mr Doria is expected to challenge Mr Bolsonaro for office in 2022 elections, and has supported development of the Sinovac vaccine, vowing to inoculate all residents of his state as early as March next year.
Earlier on Monday before Anvisa announced the trial suspension, Mr Doria said that workers had broken ground on a facility that will produce 100 million doses annually of the Sinovac vaccine. Sao Paulo will import 120,000 doses of the vaccine, expected to arrive Nov. 20.
Butantan, the Brazilian institute partnering with Sinovac, is also supported by the state of Sao Paulo.

Mr Bolsonaro has instead supported the Oxford-AstraZeneca vaccine, pledging in August to set aside 1.9 billion Brazilian real (£267.5 million) to purchase and produce those doses when they become available.
In October, he announced that Brazil would not buy the Sinovac vaccine just one day after health minister Eduardo Pazuello said the Chinese firm’s vaccine would be included in the national immunisation programme.
Mr Bolsonaro, a longtime China sceptic, has dismissed the Sinovac vaccine as lacking in credibility. On Tuesday morning, he said on his Facebook page that the suspension was "another victory for Jair Bolsonaro."
Sinovac’s vaccine has already been widely used in China, even before clinical trials conclude as part of a government emergency programme.
Hundreds of thousands have been inoculated, raising concern among experts that the health and safety risks of vaccines administered outside of clinical trials would be challenging to document.
These worries have been heightened as vaccine development – a process that normally would take years – has been squeezed into months as countries seek to contain a pandemic that has infected more than 50 million globally.

Sinovac, headquartered in Beijing, has insisted its two-shot coronavirus vaccine is indeed effective and safe and that there have been no significant adverse events. Outside of Brazil, Sinovac is also being trialled in Turkey and Indonesia.
On Tuesday, a spokesman for Indonesia’s state-owned firm, BioFarma, said that trials were “going smoothly” and that there were no plans to cancel them after the Brazil suspension.
Chinese vaccine developers have been pushing forward in hopes of winning the global race to immunise the public against the coronavirus – a move, if successful, that would help Beijing deflect public anger against its cover-up of the pandemic. China has four vaccines in late-state human trials, more than any other country in the world.


