Why China could be poised to win the race for a coronavirus vaccine

There are many ways in which the coronavirus pandemic has exposed the weakness of the West, and this week China moved up a gear in the pivotal area of vaccine diplomacy.
A string of positive announcements from Beijing contrasted sharply with the mood in the West, which was dominated by the news the AstraZeneca-Oxford vaccine trial had been briefly paused following a suspected adverse reaction in a British volunteer.
Scientists are now, perhaps for the first time, seriously considering whether China might be first to develop an effective vaccine (see graphic below).
Diplomats, meanwhile, are turning their attention to what that might mean for geopolitics in the difficult winter months ahead. It could make the flare-ups over China's exports of face masks and ventilators during the early stages of the pandemic look like minor spats.
In truth, China has been at or near the front of the Covid-19 vaccine race from the off. Of the nine candidates in Phase Three trials, four are Chinese.
And while the leading western candidates – Oxford-AstraZeneca, BioNTech-Pfizer and Moderna – have all won plaudits for their use of state-of-the-art technology platforms, experts are starting to wonder whether China's strategy of focusing on "old school" vaccine technologies may eventually prove to be more prudent.
"Three of the four Chinese candidates use inactivated Sars-CoV-2 virus which ultimately may prove to be the best bet," said Dr Vipul Chowdhary, technical lead at leading biomedical think tank Policy Cures Research.
"All they have done is basically disable the virus at the same time as maintaining its antigen properties. It is the traditional method. So it should normally provide good defence and pose less potential for reactions compared to the others."
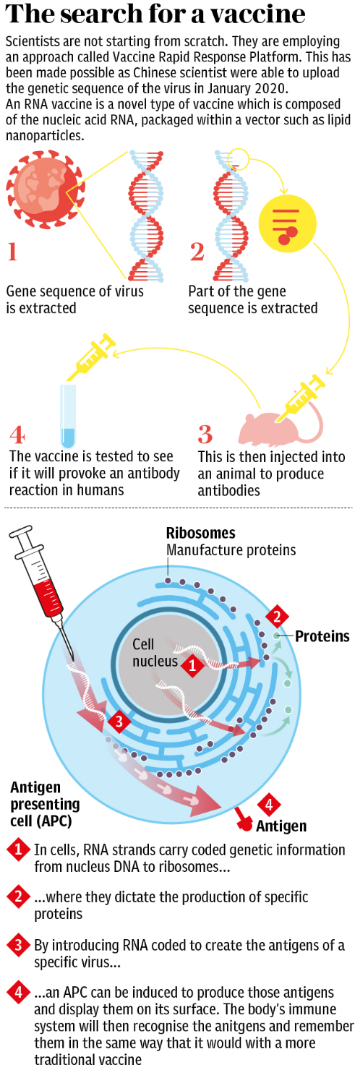
The graphic below shows the steps in the search for a vaccine:
Dr Paul Offit, the director of the Vaccine Education Center at Children's Hospital of Philadelphia, and the co-inventor of the rotavirus vaccine, agreed.
"Instinctively I feel a little more comfortable with inactivated vaccines because we have a lot of experience with them," he said. "The other advantage is that you're making an immune response to all four of the coronavirus proteins, not just the spike protein."
A first claim of efficacy
This week, China National Biotec Group (CNBG), a state-run vaccine company, said early data from its Phase Three trials showed that its two leading immunisations were effective in preventing volunteers contracting Covid-19 – the first time a claim of efficacy has been made.
Zhou Song, the secretary for the commission for discipline inspection with CNBG, told China National Radio on Monday that "hundreds of thousands have taken the shot and no one has shown any obvious adverse effects or got infected". He added that the company's two vaccines were likely to protect people for up to three years.
Dr Chowdhary cautioned that such "unverified claims mean nothing – only an adequately designed Phase Three trial showing a clear and statistically significant benefit in the intervention arm compared to placebo can prove the effectiveness".
Nevertheless, China, like others, is getting into the vaccine diplomacy game. It is using its early success to amplify the country's political influence, restore frosty relationships and further promote an image of the nation as a global health leader.
Bangladesh, where vaccine manufacturer Sinovac Biotech is testing its jab, will receive roughly 110,000 free doses if the shot proves successful, while China is offering Latin American and Caribbean nations $1 billion in loans to buy its vaccines.

In south-east Asia, China has told countries including the Philippines, Thailand, Cambodia and Vietnam that they will gain priority access to any future vaccine.
And in Africa – where China, Europe and the United States are wrestling for influence – President Xi Jinping said during a summer summit that the continent "will be among the first to benefit" once its Covid-19 vaccines are completed.
Critics point out that such largesse will undoubtedly come with strings, explicit or otherwise. Remaining silent about Beijing's territorial claims in the South China Sea and its treatment of its minority ethnic and religious groups are almost certainly a prerequisite.
Others say China's bid to become a powerhouse in global health is being aided by US President Donald Trump's withdrawal from the World Health Organisation (WHO) in particular and the world stage more generally.
Mr Trump has flatly refused to join Covax – a WHO initiative to share vaccines globally – leaving the way clear for China to play a leading role if it joins before the final participants are expected to be announced on Friday.

Some commentators fear his "vaccine nationalism" may also see countries use a Chinese vaccine even if good data on safety and efficacy is lacking.
Dr Paul Offit added that if China or Russia are first to license a jab, political pressure may mount on regulators elsewhere to push through early approval.
But not all the cards are stacked in China's favour.
Quite apart from political suspicion, China, like others, has been rocked by vaccine safety scandals in the past, and its regulatory system is opaque and may not inspire confidence.
"I think scientists and the public don't trust China, just like they don't trust Russia," said Dr Offit. "They don't trust the vaccine data, just like they still don't trust the [coronavirus] case and death numbers."
The logistical problems that stand in the way
There are serious logistical issues, too. China's success in suppressing the epidemic early on means that, like others, it has had to look further afield to set up Phase Three trials, including in the United Arab Emirates, Bangladesh (where this is little transmission of the virus) and some 9,000 health workers in Brazil.

Manufacturing and distribution could prove to be another sticking point. While traditional inactivated vaccines are less complex, said Dr Chowdhary, the manufacturing process is slowed by the need for high security labs to grow live virus at the start of the process.
Also, China does not have a large scale and globally established vaccine export business like India, for example.
Even its domestic manufacturing capability is unclear. CNBG says it has constructed a new factory, doubling its capacity to more than 200 million doses a year, while Sinovac has a new plant in Beijing capable of producing roughly 300 million doses annually – but neither is enough to cover the country's entire population.
The risk of antibody dependent enhancement
There is one great leveller in the vaccine race.
Vaccine makers of all nationalities face one particular significant hurdle, the spectre of which was raised when the Oxford vaccine was suspended last week: there is a risk that the antibodies created by a vaccine interact with those naturally acquired to spark a potentially dangerous adverse reaction. This is known as antibody dependent enhancement (ADE).
The problem for vaccine makers is testing for it. In most, perhaps all, the trials run to date, volunteers are screened ahead of time to check they have not got existing SARs-CoV-2 antibodies before being given a jab. They are then monitored for adverse reactions if and when they come into contact with the natural virus.
But Dr Chowdhary said there was a theoretical risk ADE could happen the other way round if someone previously exposed to the virus was then inoculated with new antibodies.
"It is only a theoretical risk. But in science, unless we can prove it's not there, we don't say it's not there. And as far as I know, we haven't done that yet," he said.


