Experts question claimed accuracy of Covid-19 saliva tests
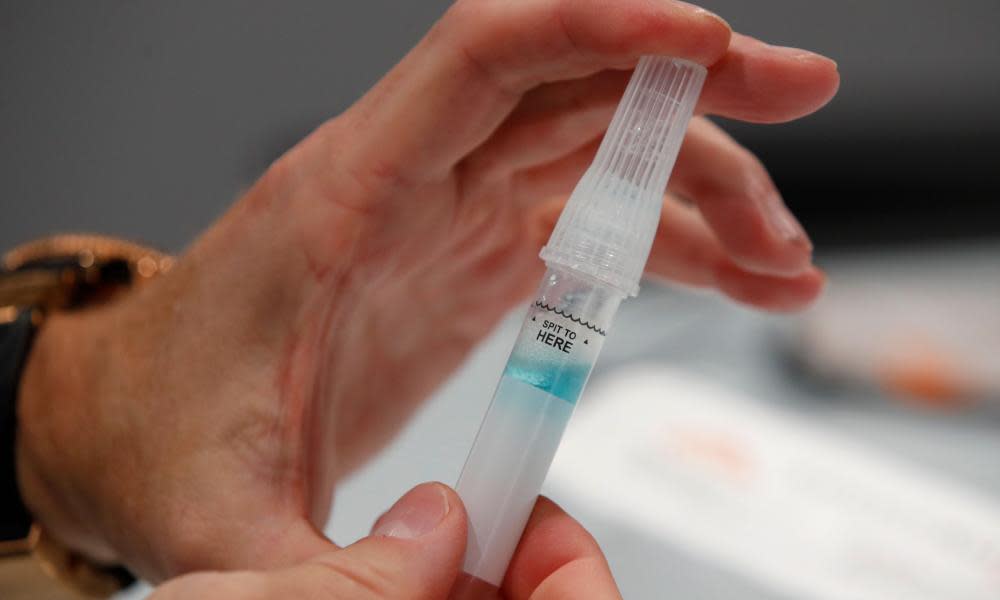
Saliva tests for Covid-19, which are being introduced for NHS workers as part of the government’s mass testing programme, pick up only 13% of people with low levels of the virus and not 91%, as the official assessment has claimed, according to experts.
Two members of the Royal Statistical Society’s working group looking at the accuracy of Covid tests have questioned the results and the way they have been evaluated.
Prof Jon Deeks from Birmingham University and Prof Sheila Bird, formerly of the MRC Biostatistics Unit at Cambridge University, say the tests perform poorly where people have low levels of the virus, which is often the case in people without symptoms.
They say the discrepancy in the figures is because the evaluation used “spiked” samples – saliva to which the virus has been added in the lab. Those manufactured samples were picked up efficiently by the test, but “real world” samples from people with asymptomatic Covid were not.
Now that the UK has authorised the first Covid vaccine, who will get it first?
The government’s Joint Committee on Vaccination and Immunisation (JCVI) says its priority is to prevent Covid-related deaths and protect health and social care staff and systems.
Elderly care home residents and their carers are first on the JCVI’s list because their risk of exposure to the virus is higher and because the risk of death closely correlates with older age. They are followed in priority by anyone else over 80 and frontline health and social care workers.
Even so, for pragmatic reasons NHS staff are likely to be the first group to receive the Pfizer/BioNTech jab. This is because the vaccine needs to be stored at ultra-cold temperatures, which can be achieved more easily by using hospital facilities
Are there enough doses to reach all the priority groups?
Together, care home residents, their carers and the over-80s make up nearly 6 million people, and frontline NHS staff a further 736,685. Matt Hancock, the health secretary, has said he expects 10m doses of the Pfizer/BioNTech vaccine to be available this year, so if this is the only vaccine authorised, everyone else would have to wait until further doses become available next year.
Where will I go for the vaccine?
Covid-19 vaccines are expected to be delivered at three types of venue: NHS trust “vaccine hubs” at hospital sites; mass vaccination centres, which are in the process of being set up at places such as football stadiums, conference buildings and racecourses – these are expected to vaccinate up to 5,000 people a day; and at GP surgeries and pharmacies. GPs can also visit care home residents and housebound patients at home without them needing to travel.
How far apart will the two doses be administered, and will I protected after the first?
While there is some evidence to indicate high levels of short-term protection from a single dose of vaccine, a two-dose schedule is what has been approved by the MHRA.
The second dose will need to be delivered at least 21 days after the first, and both will be injected into the deltoid muscle – the thick triangular muscle we use to raise each arm.
For the Pfizer vaccine, its efficacy rate was calculated seven days after the second shot. It is likely that people will have some protection before this, but this is how long it will take for full protection to kick in. We will learn more about the extent of protection and how long it lasts as data from ongoing clinical trials comes in.
Can I pay to get the vaccine privately?
Unlikely. England’s deputy chief medical officer, Jonathan Van-Tam, has said he believes Covid-19 vaccines should be delivered according to clinical priority rather than allowing people to jump the queue if they can afford it.
Will I be able to choose which vaccine I have?
Also unlikely, at least in the short to medium term. Assuming more than one vaccine is approved, the priority will be distributing any available doses to the people who need it as quickly as possible.
The OptiGene RT-Lamp test, which can be used with saliva samples or nose swabs, was intended to be part of the Liverpool mass testing programme, routinely offered to asymptomatic NHS workers. People in the community have been given lateral flow tests, which involve nose and throat swabs. Both tests have the advantage of speed, with results within half an hour.
The accuracy of the Lamp tests came into question last month, however, when a letter emerged from scientists with Greater Manchester’s mass testing expert group (MTEG). They said they found only 47% accuracy and warned that the tests should not be widely used in care homes and hospitals.
But on Monday, the government said the Lamp tests had passed an evaluation with 79% overall accuracy, which included high and low virus levels using both swabs and saliva samples. Most of the testing took place in a pilot in Southampton, where 55,000 people in the NHS and at the university took part.
The health minister Lord Bethell said the Optigene Lamp tests’ sensitivity had been confirmed in the lab and in the field.
But Deeks said the data released on Monday showed that spiked samples had been used to increase the number of samples where the virus levels were low. Using laboratory-made “spiked” samples did not reflect performance in the real world and meant that some people would wrongly think they were in the clear when they had the virus, he said.
“Before the government decides that these tests are to be used in the population, it is really important that we find out how well they work in people like us. This study has mixed together real clinical data with data from samples that have been made in the laboratory. This has led to its performance on saliva looking better than it is, and wrongly suggests that it can detect Sars-CoV-2 in samples with less virus in them,” he said.
The Pfizer/BioNTech Covid jab is an mRNA vaccine. Essentially, mRNA is a molecule used by living cells to turn the gene sequences in DNA into the proteins that are the building blocks of all their fundamental structures. A segment of DNA gets copied (“transcribed”) into a piece of mRNA, which in turn gets “read” by the cell’s tools for synthesising proteins.
In the case of an mRNA vaccine, the virus’s mRNA is injected into the muscle, and our own cells then read it and synthesise the viral protein. The immune system reacts to these proteins – which can’t by themselves cause disease – just as if they’d been carried in on the whole virus. This generates a protective response that, studies suggest, lasts for some time.
The two first Covid-19 vaccines to announce phase 3 three trial results were mRNA-based. They were first off the blocks because, as soon as the genetic code of Sars-CoV-2 was known – it was published by the Chinese in January 2020 – companies that had been working on this technology were able to start producing the virus’s mRNA. Making conventional vaccines takes much longer.
Adam Finn, professor of paediatrics at the Bristol Children’s Vaccine Centre, University of Bristol
Both the Lamp tests and the lateral flow tests were part of Operation Moonshot, of which the Liverpool programme is the pilot for the whole country. The government’s hope is to use the saliva tests in care homes, hospitals and universities.
Bird said tests should be tried out in the context in which they are going to be used. Early on in the development of a test, she said, you would use spiked samples to assess how well it was doing. “Then you gradually move closer and closer to the real world and it is the real world that matters. If you are using the test very widely and helping people to make decisions about their own lives and the lives of their relatives, you want to know about its performance in the context of use.”
A Department of Health and Social Care spokesperson said: “With up to a third of individuals with Covid-19 not displaying symptoms, broadening testing to identify those showing no symptoms and who can infect people unknowingly will mean finding positive cases more quickly and break chains of transmission.
“We are committed to using the latest testing technology, and the country’s leading scientists have rigorously evaluated the Optigene Lamp test in the lab and in the field and confirmed its sensitivity for asymptomatic testing.
“The use of spiked samples is a perfectly valid and standard practice for a study of this sort; it is in accordance with the guidelines laid down by the Technical Validation Group. The inclusion of spiked samples does not change the conclusions made”.
OptiGene was approached for comment but made no response.


