Hope for elderly as Oxford University COVID-19 vaccine produces immune response in young and old
Watch: Vaccine produces immune response, says AstraZeneca
There is renewed hope for elderly patients after tests showed a COVID-19 vaccine produced a similar immune response in both younger and older people.
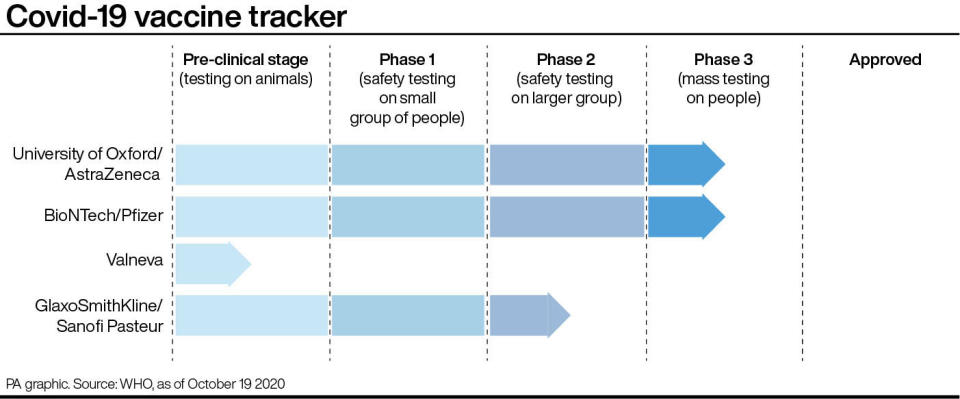
The vaccine, being developed by the University of Oxford with drugs firm AstraZeneca, could be a crucial weapon in the battle to halt the spread of coronavirus.
Adverse responses to the vaccine were lower among the elderly, tests on the vaccine revealed.
Because the immune system weakens with age, it had been feared that the elderly might not respond as positively to the vaccine.
However, an AstraZeneca spokesman told the Reuters news agency: "It is encouraging to see immunogenicity responses were similar between older and younger adults and that reactogenicity was lower in older adults, where the COVID-19 disease severity is higher.”
Referring to the technical name of the vaccine, the spokesman added: “The results further build the body of evidence for the safety and immunogenicity of AZD1222.”
On Monday, the Financial Times reported that the vaccine triggers protective antibodies and T-cells in older age groups.
Read more: 'More than one' coronavirus vaccine will be ready in early 2021, Sage expert says
The vaccine is expected to be one of the first among the large pharmaceutical companies to gain regulatory approval, along with another being developed by Pfizer and BioNTech.
Immunogenicity blood tests carried out on a subset of older participants had similar results to data released in July that showed the vaccine generated "robust immune responses" in a group of healthy adults aged between 18 and 55, the Financial Times reported.
Health secretary Matt Hancock has said a vaccine will not be widely available until some time in the first half of next year, although he did not rule it out for NHS staff before Christmas.
"We want to be ready in case everything goes perfectly but it's not my central expectation that we'll be doing that this year, but the programme is progressing well, we're not there yet," he said.
The top infectious disease specialist in the US, Dr Anthony Fauci, told BBC’s The Andrew Marr Show on Sunday that whether there is a safe and effective coronavirus vaccine will be known by the end of the year.
“We will know whether a vaccine is safe and effective by the end of November, the beginning of December,” he said.
“But the question is, once you have a safe and effective vaccine, or more than one, how can you get it to the people who need it as quickly as possible?

“The amount of doses that will be available in December will not certainly be enough to vaccinate everybody – you’ll have to wait several months into 2021.”
He said healthcare workers will likely be prioritised first for any vaccine, as well as people considered at increased risk of complications.
Dr Fauci added: “That could start by the end of this year, the beginning of January, February, March of next year.”
Watch: Can you catch coronavirus twice?
Coronavirus: what happened today
Click here to sign up to the latest news and information with our daily Catch-up newsletter



